
Aqueous CO2 solutions: equilibria and rates
 |
Aqueous CO2 solutions: equilibria and rates |
Two of the simplest molecules on Earth, carbon dioxide and water, are two of the most important (and have fascinating chemistry). Essentially all life on Earth depends on the food produced by photosynthesis, which uses light from the sun to drive the reaction between CO2 and H2O to form carbohydrates. Complex organisms also depend on the oxygen produced as a “by-product” of photosynthesis. Equilibria and rates involving carbon dioxide in aqueous solution play a central role in Earth’s carbon cycle, ocean chemistry, and climate change and are of vital importance in biological systems. This is an exploration of some of these phenomena that will be presented by the instructor and analyzed in whole-group discussion as it proceeds. |
||||
|
||||
|
||||
1.
|
What is the indicator color in the acidic solution? In the basic solutions? The indicator color change occurs in the range pH 6 to 7. Are your observations consistent with this property of the indicator? Explain your response. |
|||
Add 50 mL 0.1 M ice-cold aqueous hydrochloric acid solution to a clean 100-mL beaker containing a magnetic stir bar. Add the same number of drops of acid-base indicator as in the acid and base samples above. |
||||
2.
|
What color is the solution? Is this what is expected? Explain why or why not. |
|||
To test the rate of reaction of sodium hydroxide solution with the hydrochloric acid solution we will rapidly add 1.5 mL of the 2 M base to the magnetically stirred acid and time any color changes that occur. (A long-stem Beral pipet with the bulb half full holds about 1.5 mL.) |
||||
3.
|
How many millimoles of acid are in the beaker to begin with? How many millimoles of hydroxide will be added? |
|||
4.
|
When the reaction is complete, what color is the solution expected to be? Explain the reasoning for your answer. |
|||
Begin stirring the solution in the beaker and prepare to time any very rapid color changes (as well as any slower ones) and then quickly add 1.5 mL of the 2 M sodium hydroxide solution to the ice-cold hydrochloric acid and indicator solution. Add the base near the wall of the beaker where the mixing is most vigorous. |
||||
5.
|
What is observed? What was(were) the time(s) for any color change(s)? Was the expectation from item 4 correct? Were any of the observations surprising? Explain why or why not. |
|||
Repeat the procedure above, starting with 0.1 M ice-cold aqueous acetic acid solution in place of the hydrochloric acid. |
||||
6.
|
How many millimoles of acid are in the beaker to begin with? How many millimoles of hydroxide will be added? |
|||
7.
|
When the reaction is complete, what color is the solution expected to be? Explain the reasoning for your answer. Information that may be needed for an explanation is that the pKa for acetic acid is 4.75 and, as was said in item 1, the pH range for the color change of the acid-base indicator is in the range of 6 to 7. |
|||
8.
|
Based on the observations with the hydrochloric acid solution and what you know about acetic acid, how, if at all, do you expect the observations with acetic acid to differ? Explain. |
|||
Begin stirring the solution in the beaker and prepare to time any very rapid color changes (as well as any slower ones) and then quickly add 1.5 mL of the 2 M sodium hydroxide solution to the ice-cold acetic acid and indicator solution. Add the base near the wall of the beaker where the mixing is most vigorous. |
||||
9.
|
What is observed? What was(were) the time(s) for any color change(s)? Were the expectations from items 7 and 8 correct? Were any of the observations surprising? Explain why or why not. |
|||
Repeat the previous procedures, starting with an ice-cold seltzer water solution in place of the hydrochloric or acetic acids. Seltzer water is a saturated solution of carbon dioxide, about 0.1 M, in water. Often, this is called a “carbonic acid,” H2CO3 or (HO)2CO, solution. |
||||
10.
|
How many millimoles of acid are in the beaker to begin with? How many millimoles of hydroxide will be added? |
|||
11.
|
When the reaction is complete, what color is the solution expected to be? Explain the reasoning for your answer. Information that may be needed for an explanation is that the first pKa usually given for carbonic acid (carbonic acid to bicarbonate ion) is about 6.4. |
|||
12.
|
Based on the observations with the hydrochloric and acetic acid solutions, how, if at all, do you expect the observations with dissolved carbon dioxide to differ? Explain your reasoning. |
|||
Begin stirring the solution in the beaker and prepare to time any very rapid color changes (as well as any slower ones) and then quickly add 1.5 mL of the 2 M sodium hydroxide solution to the ice-cold dissolved carbon dioxide and indicator solution. Add the base near the wall of the beaker where the mixing is most vigorous. |
||||
13.
|
What is observed? What was(were) the time(s) for any color change(s)? Were the expectations from items 11 and 12 correct? Were any of the observations surprising? Explain why or why not. |
|||
In solutions of carbon dioxide dissolved in water, most of the carbon dioxide is present as the aquated molecule, CO2(aq), in equilibrium with a small amount of hydronium ion and hydrogen carbonate (bicarbonate) ion. |
||||
14.
|
What evidence is there from the results in item 13 about the rate of the forward reaction in this equilibrium? (Is formation of hydronium ion slow or fast?) Explain how the evidence supports this conclusion. |
|||
Repeat the seltzer water (dissolved CO2) procedure with a variation. Add 50 mL 0.1 M ice-cold aqueous seltzer water to a clean 100-mL beaker containing a magnetic stir bar and stir in a few drops of blood or equivalent. Add the same number of drops of acid-base indicator as in the acid samples prepared above. |
||||
15.
|
What color is the solution? Is this what is expected? Explain why or why not. |
|||
16.
|
When the reaction is complete, what color is the solution expected to be? Explain the reasoning for your answer. |
|||
Begin stirring the solution in the beaker and prepare to time any very rapid color changes (as well as any slower ones) and then quickly add 1.5 mL of the 2 M sodium hydroxide solution to the ice-cold dissolved carbon dioxide, blood, and indicator solution. Add the base near the wall of the beaker where the mixing is most vigorous. |
||||
17.
|
What is observed? What was(were) the time(s) for any color change(s)? How did the observations compare to those made in item 13? Were any of the observations surprising? Explain why or why not. |
|||
18.
|
What evidence is there about the rate of the forward reaction in the above equilibrium in the presence of blood in the sample? Explain how the evidence supports the conclusion. |
|||
 |
||||
Instructor/presenter notes |
||||
The objective of this activity is to observe the difference between hydroxide reacting with CO2 dissolved in water compared to the reaction with other acids and use the results to draw conclusions about the rates and equilibria for the reaction of CO2 with water. The biological implications for metabolic CO2 in the bloodstream are also examined. |
||||
1.
|
The indicator is yellow in acid solution and blue in base solution. This is consistent with an indicator color change in the range pH 6 to 7 with different colors, yellow at acidic pH and blue at basic pH. The indicator is green (a combination of transmitted blue and yellow light) in solutions at slightly acidic pH. |
|||
2.
|
The hydrochloric acid solution is yellow, as expected for an acidic solution. |
|||
3.
|
The millimoles of solutes in solutions are the product of the volume of the solution in milliliters and its molarity. millimoles HCl = (50 mL)(0.1 M) = 5 mmol millimoles NaOH = (1.5 mL)(2 M) = 3 mmol |
|||
4.
|
In the reaction, one millimole of acid reacts with one millimole of base, so the base will be used up and 2 millimoles of acid will be left in solution. The final solution will be acidic and be yellow, the indicator color in acid. |
|||
5.
|
Usually a flash of blue color appears where the base is added to the solution near the edge of the solution (with no perceptible change in the yellow color of the bulk solution). The color disappears faster than can be timed by eye and a watch, although students often suggest times of a second or two, which can be accepted as “very fast”. Generally, no one is surprised by the speed of the reaction between the (completely ionized) strong acid and strong base. |
|||
6.
|
As in item 3, there are 5 mmol of acid to which 3 mmol of base will be added. |
|||
7.
|
When the acid-base reaction is complete, the solution will contain 2 mmol acetic acid and 3 mmol acetate ion produced by the reaction. This is a buffer solution that should have a pH a bit on the base side of 4.75 (the acid pKa), but well below pH 6. The solution will be yellow, since pHs below 6 are on the acid side of the indicator range. |
|||
8.
|
Since acetic acid is a weak acid, not completely ionized, students often predict that the reaction will be slower (requiring time to ionize) than with the strong acid. |
|||
9.
|
There is no perceptible difference between this reaction and that with the strong acid. The reaction is still “very fast” and pretty much impossible to quantify by eye. If a prediction of a slower reaction was made, the result is a bit surprising, but can be explained by assuming the forward and reverse equilibrium, |
|||
The acetic acid solution is used in this activity to show that the neutralization of most weak acids is very rapid. Essentially the same result is obtained for 0.1 M hydrochloric acid (or other strong acid) as for the acetic acid, since any basic indicator color observed transiently in the solutions is due to inefficient mixing, not the rate of the chemical reaction. This is an important point to establish, since students often think that a weak acid will give up its protons slowly enough to be observed on a time scale of seconds or minutes. There is no observable difference between the reaction carried out at room temperature or with ice-cold acids, but these acids are used cold to be sure students do not think that temperature is the factor causing the slow change in the case of the dissolved carbon dioxide (seltzer water) solution. (This Activity is described in reference 1.) |
||||
10.
|
As in items 3 and 6, there are 5 mmol of acid to which 3 mmol of base will be added. |
|||
11.
|
Although, as discussed below, there is essentially no carbonic acid in a dissolved carbon dioxide solution, we can reason about the end result of the acid-base reaction as though this equilibrium exists, |
|||
12.
|
Carbonic acid, with a pKa of 6.4, would be a weak acid, somewhat weaker than acetic. Based on the previous observations (in the absence of any other knowledge of dissolved carbon dioxide solutions), predicting the same sort of behavior, rapid discharge of the blue color when base is added, would be logical. |
|||
13.
|
The solution turns uniform blue and remains blue for many seconds—usually 20 to 40, depending on the exact conditions (temperature, concentrations). Watching for the end of the reaction takes some attention. The change from blue to the final color green (as predicted) is not instantaneous, but usually takes a few seconds. Those paying close attention see the beginning of the change and generally call out, so everyone is aware it is happening. The striking difference in time for the reaction is always surprising and unexpected after the first two acids. |
|||
14.
|
The explanation for the observed slow reaction of hydroxide with a solution of carbon dioxide dissolved in water must be that the rate of formation of hydronium ion from dissolved carbon dioxide is slow. |
|||
A likely mechanism, based on the electron distribution in the molecules, is |
||||
|
||||
The electronegative oxygen atoms draw electron density away from the central carbon atom in carbon dioxide. The resulting partial positive charge on the carbon and the partial negative charge on the oxygen in a polar water molecule attract one another to form the acidic intermediate that rapidly transfers a proton to another water molecule. The net result of these reactions is the equilibrium reaction written previously, The reaction mechanism is a bit different at pH > 9, which corresponds to the solution formed in the first instant at the place where base is added in this demonstration. The fast, direct reaction of hydroxide ion accounts for most of the reaction of carbon dioxide. |
||||
|
||||
The pH of the solution falls rapidly during this stage of the reaction. When the pH falls below about 9, this second order pathway becomes unimportant (at ice temperature). Hydronium ion continues to be formed slowly from the remaining dissolved carbon dioxide. Thus, the pH continues to fall toward the equilibrium value for the hydrogen carbonate/dissolved carbon dioxide buffer system, but it falls much more slowly because the rate-determining step is now the slow hydration of the dissolved carbon dioxide. Since the pH is still above the indicator color change point, the solution is still blue. Jones, Haggett, and Longridge have devised an interesting “double-clock” experiment, in which both the rapid and slow stages of this overall process are studied (3). These authors also suggest a demonstration of the slow stage that is very similar to that described here. The indicator chosen to signal the "end point" of these neutralization reactions should have a color transition in the pH range between 6 and 7, because the final hydrogen carbonate/dissolved carbon dioxide buffer system has a pH near 6.4, the pKa for carbonic acid (actually the final equilibrium mixture of dissolved carbon dioxide, hydronium ion, and hydrogen carbonate). Bromothymol blue (pH range 6.0 – 7.6) is an excellent, readily available candidate for this task. |
||||
15.
|
The solution is usually sort of orange, a combination of the yellow indicator and the light red from the added drops of blood (liquid extracted from minced red meat). |
|||
16.
|
Assuming that the final reaction mixture is a buffer of dissolved carbon dioxide and bicarbonate, the indicator will give a green color mixed with the red from the blood, so the color will be a bit “muddy”, but should still be easily distinguishable from the base solution blue. |
|||
17.
|
The time for the color change is usually less than half what was observed without the blood. At this point in the Activity, this is less surprising than other observations, since there was really no expectation about what might happen, except that something probably would be different. Why else would we do this odd thing (adding the blood)? |
|||
18.
|
Since the reaction reaches its “end-point” (the buffer mixture) faster, this must mean that the hydronium ion is formed faster to get to this point. Something in the blood must speed up the reaction of the dissolved carbon dioxide with water to form bicarbonate and hydronium ions. |
|||
Carbon dioxide is produced in our cells as a result of the respiratory processes that provide us energy from our fuel (food). It is carried away in the blood (mainly as bicarbonate), released as carbon dioxide gas in the lungs, and finally exhaled. Getting rid of this end product of oxidation of our fuel molecules is important, because its build up would quickly lead to acidosis (and death). However, the uncatalyzed dehydration reaction, (HO)CO2–(aq) + H3O+(aq) → CO2(aq) + H2O(l) the reverse of the hydration reaction, is also very slow (1, 2). If there were not some mechanism for speeding up these reactions, life, as we know it, would not be possible. Fortunately, blood contains an enzyme, carbonic anhydrase, that catalyzes these reactions. (If you know the history of the Apollo 13 mission to the Moon or have seen the movie about this mission, you may recall that the crew faced a potentially fatal situation when the device that scrubbed CO2 from the air in the cabin failed and they were in danger of dying from the build-up of CO2 in their blood.) |
||||
 |
||||
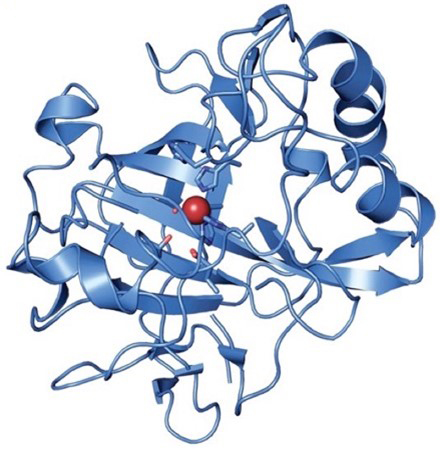
This is a representation of carbonic anhydrase, a relatively small metalloprotein (a protein containing a metal ion, maroon ball, as part of its structure—zinc in this case) with a molar mass of about 30,000 daltons. The zinc ion is essential to the enzyme’s function; its removal causes the enzyme to lose its activity. In this traditional representation of a protein structure, the blue string and ribbon show how the amino acid protein chain folds on itself to form the active structure of the enzyme. Some of it looks like random strings, but there is an α-helical portion at the upper right and series of β-sheets at the lower left that are associated with the metal ion. Near the metal ion, a few of the side groups from the amino acid chain are shown as stick structures. These are often shown if they have functional polar parts (like -COOH, and -NH2) that take part in the catalytic reaction (interacting directly with the reactant molecules). The enzyme is inhibited by thiocyanate ion; the time for neutralization in the presence and absence of thiocyanate can be measured to test this assertion (4). Carbonic anhydrase has one of the highest activities of any enzyme known. One enzyme molecule can hydrate 105 molecules of CO2 per second, which is 107 times faster than the uncatalyzed reaction (5). This high activity is required, if the dehydration of bicarbonate is to be rapid enough to get rid of most of it in the residence time of only seconds that our blood has in the lungs. One can get a sense of how efficient the catalyst is from the observation that a few drops of liquid extracted from red meat in about 50 mL of reaction mixture in this Activity (a dilution of more than 100-fold) usually decreases the time required for the neutralization by a factor of at least two to three. Your blood is not the only place carbonic anhydrase appears in your body. There is a family of carbonic anhydrase enzymes with different structures and different roles. For example, the tingle you feel from the fizziness of a carbonated beverage requires a carbonic anhydrase associated with the taste sensor on your tongue that creates the tingly sensation. The enzyme catalyzes the reaction studied in this activity to produce hydronium ion from the dissolved carbon dioxide in the drink and the hydronium ion triggers the sensor to send its tingly message to the brain. Another member of this enzyme family is found in the aqueous humor, a watery fluid between the cornea and lens of your eye. It catalyzes the hydration of carbon dioxide that dissolves from the atmosphere and the hydronium ion formed gets exchanged for sodium ion, which helps form the aqueous humor. In some forms of the eye disease, glaucoma, pressure builds up inside the eye and can harm its internal structures. One contributor to the build-up of pressure is the formation of too much aqueous humor. A medication often used in the treatment of glaucoma is a solution of a drug that inhibits the activity of carbonic anhydrase and reduces the formation of aqueous humor. |
||||
| Carbonic acid | ||||
Although there is essentially no carbonic acid, (HO)2CO, in an aqueous solution of carbon dioxide, the compound can be synthesized and studied. An excellent review of results of research on the synthesis structure, and properties of carbonic acid is available (6). Because carbon dioxide and water are such stable molecules, the dissociation of carbonic acid is favored thermodynamically. However, even though the dissociation is highly exothermic, quantum mechanical calculations show that there is a large activation energy barrier for dissociation. Thus, it has been possible to synthesize carbonic acid at low temperatures and study its spectrum. In theory, the dissociation reaction should be slow enough to study the molecule at room temperature, but the reaction is catalyzed by surfaces and other molecules, including water. Even a single water molecule lowers the activation energy barrier and the lifetime of carbonic acid formed in aqueous solution by ultrafast protonation of bicarbonate ion is only a few hundred nanoseconds. This certainly explains its absence in aqueous solutions of carbon dioxide. |
||||
References |
||||
1.
|
Shakhashiri, B. Z., “Carbon Dioxide Equilibria and Reaction Rates: Carbonic Anhydrase-catalyzed Hydration,” Chemical Demonstrations, Vol. 2, (University of Wisconsin Press, Madison, WI, 1985), 122-127. |
|||
2.
|
Kern, D. H., “The Hydration of Carbon Dioxide,” J. Chem. Educ., 1960, 37, 14 -23. |
|||
3.
|
Jones, P., Haggett, M. L., and Longridge, J. L., “The Hydration of Carbon Dioxide: A Double Clock Experiment,” J. Chem. Educ., 1964, 41, 610 - 612. |
|||
4.
|
McGeachin, R. L., “Inhibition of Carbonic Anhydrase by Thiocyanate: A Demonstration,” J. Chem. Educ., 1955, 32, 191 - 192. |
|||
5.
|
Stryer, L., Biochemistry, 1st ed. (W. H. Freeman and Company, San Francisco, 1975), 115. |
|||
6.
|
Bucher, G., and Sander, W., “Clarifying the structure of carbonic acid,” Science, 2014, 346(6209), 544-545. |
|||
Reagent preparations |
||||
0.1 M hydrochloric acid: Add 0.8 mL of concentrated, 12 M, hydrochloric acid to 100 mL of water and mix well (or dilute an available hydrochloric acid solution to give a 0.1 M solution). Store in a labeled bottle. 0.1 M acetic acid: Mix 10 mL of white vinegar with 80 mL of water. Store in a labeled bottle. Seltzer water: An ice-cold, unopened can or bottle from the supermarket seems to work well. This solution is made under higher than atmospheric pressure of carbon dioxide, so bubbles begin to come out immediately when the can or bottle is opened. These are apparent in the solution used, but do not interfere with seeing the colors. (This demonstration also works with club soda, but the solution contains mineral salts, in addition to carbon dioxide. Your audience might think the results are due to their presence, not the properties of the dissolved gas, so it’s pedagogically best to use seltzer.) 2 M sodium hydroxide solution: Add 0.80 g sodium hydroxide pellets to 5 mL distilled water—caution, a great deal of heat is evolved—and allow the solution to cool to room temperature; dilute the resulting solution to 10 mL. Store in a labeled, tightly capped plastic bottle. Bromothymol blue (3', 3 "-dibromothymolsulfonaphthalein) acid-base indicator solution. Dissolve 0.1 g indicator in 25 mL 0.01 M sodium hydroxide and dilute the resulting solution to 250 mL. Store in a labeled bottle. Blood may be obtained from any mammalian source for this demonstration. Fresh red meat such as beef or liver is a safe source. Puree a small sample of meat. Filter the puree through filter paper, perhaps in a small Büchner funnel, so the mush can be pressed to get the liquid out. Alternately, a bit of the puree can be used. Although this is a bit distracting in the stirred solution, it does not interfere with observation of the color change. The puree can be frozen and small chunks of it unfrozen and used for more trials. Although unlikely, you might be in a situation where you may use human blood. Outdated whole human blood from a blood bank is a source, but may not be allowed for use outside medical settings. |
||||
 |
||||
To obtain a Word file of this Activity, please fill out this brief form to help us track what is happening to our Workbook. We also encourage you to get in touch if you have an activity or idea for an activity that might add to the Workbook. We want to make this an alive and active document. |
||||
 |