www.scifun.org |
||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||
Fireworks! |
||||||||||||||||||||||||||||||||||
Blue Sky Science: How do fireworks get their color and shape?
|
||||||||||||||||||||||||||||||||||
Have you ever been to an aerial fireworks show at an amusement park, baseball game, Fourth of July celebration, or on New Year's Eve and wondered about how all the impressive colors and sounds are produced? People everywhere enjoy the fantastic explosions and the brilliant light displays of fireworks. However, these spectacles are much more than just a form of entertainment. Each firework launched into the sky is a precisely formed assembly of chemicals and fuel, carefully calibrated to produce a particular effect – a red chrysanthemum spray accompanied by a powerful explosion, or a blue strobe, for example. Understanding how the contents of a firework produce the impressive variety of colors, forms, and sound intensities requires only a simple understanding of chemical reactions.
Fireworks generate three very noticeable forms of energy: a tremendous release of sound, bright light, and heat. The tremendous booms heard at ground level are the result of the rapid release of energy into the air, causing the air to expand faster than the speed of sound. This produces a shock wave, a sonic boom.
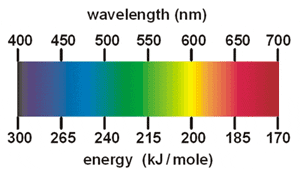
The colors are produced by heating metal salts, such as calcium chloride or sodium nitrate, that emit characteristic colors. The atoms of each element absorb energy and release it as light of specific colors. The energy absorbed by an atom rearranges its electrons from their lowest-energy state, called the ground state, up to a higher-energy state, called an excited state. The excess energy of the excited state is emitted as light, as the electrons descend to lower-energy states, and ultimately, the ground state. The amount of energy emitted is characteristic of the element, and the amount of energy determines the color of the light emitted. For example, when sodium nitrate is heated, the electrons of the sodium atoms absorb heat energy and become excited. This high-energy excited state does not last for long, and the excited electrons of the sodium atom quickly release their energy, about 200 kJ/mol, which is the energy of yellow light.
The amount of energy released, which varies from element to element, is characterized by a particular wavelength of light. Higher energies correspond to shorter wavelength light, whose characteristic colors are located in the violet/blue region of the visible spectrum. Lower energies correspond to longer wavelength light, at the orange/red end of the spectrum.
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
The colors you see exploding in the sky are produced by the elements with the characteristic emissions listed in the table below:
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
In
making fireworks, the metal salts are put into stars, small clay or dough-like
lumps or cubes 3 to 4 cm in diameter. Stars consist of a blend of oxidizing
agent, reducing agent, coloring agent (metal salt), and binders. When ignited,
the stars produce both sound and light effects. The appearance of a firework
is determined by its stars, which are made by hand and carefully packed into
cardboard compartments within the firework shell, where they await ignition
by a time-delay fuse.
Fireworks are classified as both a low and a high explosive. The initial lift charge that sends the firework into the sky is a low explosive. The burning charge undergoes rapid decomposition, but not detonation. The firework can be thought of as flying through the air powered by a fast burning wick. Where the wick ends, it meets the high explosive components of the firework. In this second stage there is an instantaneous detonation producing both a loud explosion and a bright flash of color.
The black powder lift-charge is calculated to exhaust itself precisely when the slow-burning, time-delay fuse reaches the first compartment packed with light-producing stars and black powder. This happens when the firework is at the very apex of its upward flight. Simultaneously the fuse sets off sound-producing explosives and detonates the stars, initiating color emission. If the timing of the fuses is off, however, the firework may detonate early, too close to the ground, or late, when the firework is falling back to earth.
|
||||||||||||||||||||||||||||||||||
| Chemistry of Fireworks | ||||||||||||||||||||||||||||||||||
The
sights and sounds of each explosion are the result of several chemical reactions
– oxidations and reductions – taking place within the firework as
it ascends into the sky. Oxidizers produce the oxygen gas required to burn the
mixture of reducing agents and to excite the atoms of the light-emitting compounds.
Various oxidizers are used in both the black powder and the stars. The most
commonly used oxidizers are nitrates, chlorates, and perchlorates. The reducing
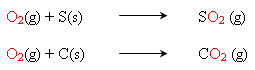
agents, sulfur and carbon, combine with the oxygen from the oxidizers to produce
the energy of the explosion.
In the 1830s Italian fireworks makers found a group of more explosive oxidizers, which produced temperatures of 1700 to 2000°C and made possible the creation of much more intense colors. These oxidizers are the chlorates, which contain the chlorate ion (ClO3-), and they give up all their oxygen upon reaction.
This results in a much more intense and spectacular reaction.
These
chlorates have the disadvantage of being less stable mechanically than nitrates,
and therefore more dangerous to handle. Chlorate compounds sometimes can be
detonated just by dropping them on the ground! This instability results from
the fact that although the chlorine atom has the potential to bond with four
oxygen atoms, in chlorates it bonds with only three, leaving the chlorine atom
unsaturated and reactive. The complete release of its oxygen atoms makes chlorate
a better oxidizing agent than nitrate. Unlike nitrate-containing compounds that
produce a relatively slow burning rate, the oxidation by chlorates produces
a much faster detonation – an explosion. In recent years, fewer fireworks
manufacturers are using chlorates. Instead, perchlorates are now more commonly
used because of their increased stability and oxygen release.
So,
perchlorates are not only more stable, but more oxygen-rich than chlorates.
They, like chlorates, produce more vigorous reactions than nitrates.
The reactions that produce these gases also release a great deal of heat energy, so not only are the gases produced rapidly, they are hot and rapidly expanding gases. This adds to the explosive force of the reaction.
|
||||||||||||||||||||||||||||||||||
| Firework Safety | ||||||||||||||||||||||||||||||||||
Fireworks are used so frequently today in celebrations that it is easy to forget that they are dangerous explosives. Every year more than 8,000 people in the U.S. suffer injuries caused by the personal use of fireworks. Nearly half of the victims are children. A third of the injuries are caused by illegally obtained fireworks, and burns account for half the injuries. (An ordinary sparkler burns at a temperature of more than 1000°C!)
The National Fire Protection Association enforces stringent safety regulations for large fireworks displays, and has valuable information on how to safely and responsibly handle consumer fireworks. Spectators must be kept at least 840 feet from the launch area (that's based on the height and burst diameter of the largest shells). Shells may not be launched if winds are stronger than 20 miles per hour, because they could be blown off course. Nevertheless, many accidents occur with unregulated, informal neighborhood displays, when spectators attracted to the activities stand dangerously close to the launch area.
Fireworks manufacturers also go to great lengths to ensure safety, but even so, more than 20 workers were killed in firework plants in the U.S. between 1970 and 1995. Safety regulations require that buildings be separated by concrete blast walls and that roofs be weakened to ensure that any explosion travels upwards rather than outwards. In addition, most fireworks are still made by hand because metal machinery could produce sparks or static electricity which would ignite the explosives.
Many animals are frightened by the noise of fireworks and people are urged to leave their pets at home when they go to fireworks displays. Sadly, there are reports of dogs running away and some were lost.
|
||||||||||||||||||||||||||||||||||
| Origins of Black Powder | ||||||||||||||||||||||||||||||||||
Gunpowder or black powder was invented in China by alchemists experimenting with a naturally-occurring salt, potassium nitrate, also known as saltpeter. Ironically, they were looking for an elixir of immortality. But in handling and heating the sensitive substance they inevitably discovered its explosive properties. The first known account of the use of gunpowder as a weapon dates to 1046 in China, describing a catapult-launched grenade, an incendiary bomb, and a smoke bomb. The Song Dynasty Emperor in 1067 banned the sale of saltpeter and sulfur to foreigners and nationalized the production of gunpowder.
Marco Polo is sometimes given credit for bringing gunpowder to Europe but that is unlikely. When Europeans invaded the Middle East during the Crusades, they encountered gunpowder weapons used by Moslem forces. Despite government control and attempts to keep the formula secret, gunpowder probably traveled the Silk Road from China to the Moslem world far earlier than Marco Polo's trip in the late 1200s. English philosopher Roger Bacon (1217-1292) is believed to the first Westerner to describe gunpowder and fireworks. By the mid-1300s, European armies were using crude cannons and other gunpowder weapons.
|
||||||||||||||||||||||||||||||||||
| Environmentally Friendly Fireworks | ||||||||||||||||||||||||||||||||||
Chemists continue to explore ways to make new pyrotechnic compounds and mixtures that are environmentally friendly. Two recent reports describe pyrotechnics made from high-nitrogen compounds that produce less smoke and particulate matter, and also replace perchlorates as oxidizers.
For more information on environmentally friendly fireworks, check out these articles from Chemical and Engineering News:
|
||||||||||||||||||||||||||||||||||
Further Reading:
Chemistry of Pyrotechnics: Basic Principles and Theory, by John A. Conkling, Marcel Decker, Inc., 1985 Beyond the Light Show: The Effects of Fireworks on Animals and People, The New York Times, 7.4.2025
|
||||||||||||||||||||||||||||||||||
| Back to Learn About | ||||||||||||||||||||||||||||||||||