www.scifun.org |
||
 |
||
 |
||
 |
||
|
Have you ever looked up at a crossing light while walking a few blocks in the city? The first thing you probably notice is whether the sign is showing that it’s safe to cross the road. But have you taken a closer look? If you’re very observant, you’ll notice that the sign contains mosaic-like patterning, pieces of which make up both the "stop" light and the "walk" light. Have you ever wondered how these types of lights work? Like many other wonderful light-up signs and devices that we see every day, the pedestrian crossing lights on street corners shine brightly thanks to the use of light emitting diodes.
Light emitting diodes, commonly called LEDs, have come to be a staple in the world of electronics. They do dozens of different jobs and are found in all kinds of devices. Some of the jobs that LEDs are used for include forming the numbers on digital clocks, transmitting information from remote controls, and letting you know when your appliances are turned on. More recently, LEDs have been utilized to provide the light needed for liquid crystal displays (LCDs) to shine in television displays and traffic lights.
|
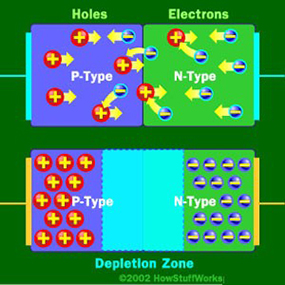
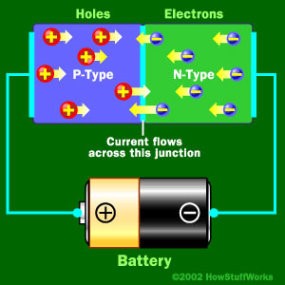
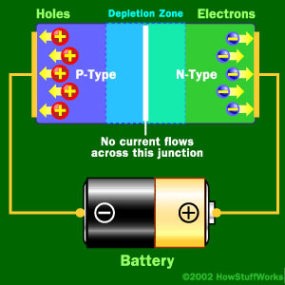
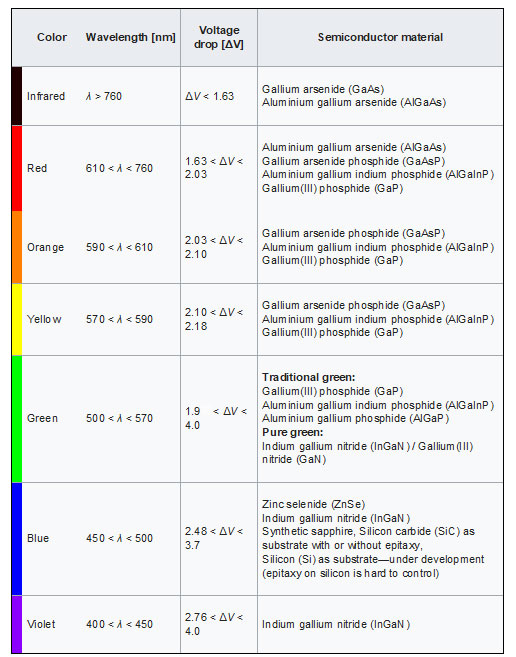
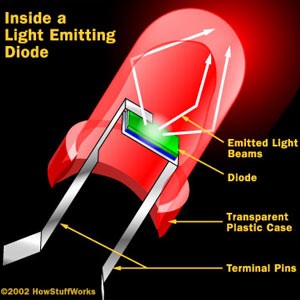
LEDs are very small light bulbs that fit easily into an electrical circuit. Most smaller multi-color LEDs (like the ones we have at Science is Fun, shown in the animation at the top of the page) are actually a set of three bulbs, arranged as pictured at the left. Together, these small lights can be turned on in different combinations to make a variety of colors shine through! LEDs are illuminated by the movement of electrons in a semiconductor material. Unlike ordinary incandescent bulbs, LEDs do not have a filament that can burn out, and don't get hot when left on for a long time. The lifespan of an LED surpasses that of an incandescent bulb by thousands of hours! These properties are some of the reasons why LEDs are already replacing the tubes that light up LCD HDTVs, making dramatically thinner televisions.
|
 Photo credit: toppr.com |