Most of the infant and adult diapers available in supermarkets and pharmacies are “superabsorbent”. In Procedure 1 of this activity you cut up some diapers, collect some of what makes them superabsorbent, and then observe superabsorbency. You will also discover why these diapers don’t leak (at least, if they are changed regularly). In Procedure 2, you can test intact diapers to see how they actually work. Unfortunately, you don’t see what is going on inside, but your discovery about leaking may be reinforced. |
|
| PROCEDURE 1 | |
The key material in these diapers is a very water-absorbent powder called (as you would expect) a “superabsorber”. Your first task is to get some of this material out of a diaper and then test to see how much water (and water containing other stuff) it can absorb. |
|
| WHAT YOU WILL NEED | |
|
|
PREPARATION |
|
Make a solution of 0.9% salt water by adding ½ teaspoon of salt to 1 ¼ cups of distilled water and mixing with a spoon. This solution has about the same amount of salt as normal human urine, so it’s a good stand-in for testing diaper absorbency. Label three zip-seal bags with a Sharpie: “Distilled Water”, “Tap Water”, and “Salt Water”. |
|
COLLECTING THE SUPERABSORBENT |
|
Do the first part of this activity on a large flat surface over a piece of dark paper or disposable table cloth so that excess material can be collected and thrown away. Unfold three diapers and place one in each of the labeled Ziploc bags. Use scissors to cut the diapers in the bags into approximately 2-3 inch squares. Some white granules may fall out of the diaper when you are cutting – this is ok. Keep the granules and the diaper material in the bags while you cut. |
 |
When you are done cutting, seal the bags, shake vigorously and then look at the corners of the bags as you tilt them to one side. Keep shaking and moving around the material until you see a pile of white granules in the corner of the bags. Placing a bag on a hard surface and gently pounding it with your fist may also help loosen some of the material. These white granules are the superabsorber. Open the bags and carefully remove everything except the white granules. Throw the diaper material you removed from the bag into the trash. It’s a good idea to wash your hands after this step. |
 |
| HOW ABSORBENT IS THE SUPERABSORBER? | |
Without opening the bags, shake the granules into one corner of each bag and estimate the volume (number of teaspoons) of the white solid. Put your estimates in a table like the one below. Now shake the granules around so that they are in a relatively even layer across the bottom of the bags. Open each bag, add one tablespoon of each type of water (distilled, tap, and salt) to the appropriately labeled bag and reseal the bags. Make sure to either use a separate spoon for each water type or dry the spoon with a napkin or towel between each addition. Gently mix the contents by rocking the bags back and forth for about 10-30 seconds. What do you observe? Feel the contents of each bag. Do all the contents feel the same? Do the contents feel like a liquid? If the contents do not all feel the same, how would you describe the differences among them? Try adding ¼ to ½ cup more of each water to the appropriate bag, seal, and mix by massaging the bags (gently squeezing and rolling the bags between your hands). Do the contents look much the same or different than before you added more water? Do the contents feel the same or different than before you added more water? If the contents do not look or feel the same, how would you describe the differences? Keep adding each water ¼ to ½ cup at a time. Massage the bags after each addition to mix the contents well and to see whether the contents feel any different when more liquid has been added. Do the three bags feel the same after each addition? Keep a careful record of the total amount of each water added to each bag. Stop adding liquid when the granules no longer appear to be absorbing it. How will you be able to tell that water is no longer absorbed? Record the total amount of water you added to each bag in a table like this: |
|
|
You can design your own data table, or click on the example above |
|
Once you are done adding water to all three bags, think about what they look and feel like and the amounts in your results table. Which bag is heaviest? Which lightest? Are these weights what you would expect from looking at the amounts in your table? How does the amount of each water absorbed compare to the amount of diaper superabsorbent in its bag? Does the name ”superabsorbent” make sense for this material? (If you like to put numbers on comparisons like this, see the “Some Arithmetic” section below.) These diapers are also called “leakproof”. Do your results help to understand why the diapers don’t leak? Does the superabsorber you tested have the same “super-ness” for all the types of water you used? If not, what is likely to cause the difference between the most absorbed and least absorbed water? Think about your three bags and a diaper on a baby. Can you think of a possible problem, if all the water samples behaved like the distilled water? Finally, try sprinkling a bit of table salt into the distilled water bag, sealing it, and massaging the bag for about a minute. Is there a change in the way the contents feel? If so, how would you describe the change? |
|
| SOME ARITHMETIC | |
| To compare the amounts of superabsorbent and water in each bag, it’s best to use the same measurement unit for the amounts. It’s usually a good idea to choose a unit that is most convenient for the smallest amount measured. In this case, teaspoons (tsp) are probably good units. Other volumes you measured were tablespoons (Tbsp) and cups. The standard relationships among these units are: 1 Tbsp = 3 tsp and 1 cup = 16 Tbsp = 48 tsp. To see how this works, let’s look at a possible example where the estimate of superabsorbent is 1-¼ tsp (a little more than one teaspoon) and the amount of water is 1 Tbsp + ¾ cup. Since ¾ cup is 12 Tbsp (three fourths of 16), the amount of water is 13 Tbsp = 39 tsp. To do the comparison, amount of water to amount of superabsorbent, it is easier to use decimals instead of fractions, because all calculators can handle decimals. So, 1-¼ tsp = 1.25 tsp and: | |
 |
|
| Thus, for this water, 31 volumes of water were absorbed for each volume of superabsorbent. If you decide to do this kind of analysis, round off your comparison ratios to the nearest whole number (as here). This activity is designed to help you understand what superabsorbents are and some of their properties, not to make very precise measurements. | |
| DISPOSAL | |
| Throw away the sealed bags in the garbage. Do not put any of the superabsorbent down a drain; it might clog the drain. | |
The superabsorbent material in the diaper that you saw as white granules is a polymer called sodium polyacrylate. A polymer is a kind of chemical compound made of many repeating parts (poly = many, mers = parts). The parts are small molecules that link together through chemical bonds, forming long chains with useful properties. The sodium polyacrylate structure is specially designed to take in and retain lots of water molecules. (One commercial form of sodium polyacrylate is, in fact, called Water-Lok.) Its polymer chains have several attachments (chemical bonds) to one another. This creates a structure you can think of as a three-dimensional net that can trap water molecules within the mesh. When your diaper superabsorbent, usually a combination of sodium polyacrylate with other absorbers, soaked up water, you found that a semi-solid gel forms (sort of like a gelatin dessert) and swells to many times the original size of the powder. These properties, gelling and swelling, are a result of water molecules entering the polymer mesh and the mesh expanding as more and more water molecules are locked inside. Since the gel is semi-solid, it tends to stay put, in a diaper as well as in your zip-seal bags. That’s why the diapers are “leakproof”. You will usually find that a diaper’s sodium polyacrylate easily absorbs lots of distilled water, about half as much tap water, and only a small amount of salt water. The reason for these differences is salt content. The polymer itself is “salty”, in the sense that the mesh contains positive ions (sodium) and negative ions (from negative groups on many of the small molecules that make up the chains). The saltiness of the polymer and any saltiness of the water being absorbed work against one another and interfere with the polymer’s ability to absorb water. Distilled water is pure water, so there is no interference with its absorption and the maximum amount can be absorbed. However, tap water contains a number of dissolved salts, lowering the polymer’s absorbing power. The EPA recommends that the total dissolved solids in drinking water not exceed 0.05% (500 parts per million, ppm). Is it surprising that such a small amount of salt can reduce the polymer’s absorbency by as much as you saw? You can see the kinds of solids dissolved in your tap water by accessing your drinking water quality report, an annual report compiled by community water systems as required by the EPA. To find the report online, search for “Annual Water Quality Report” and your city/state. The salt water you used was 0.9% table salt (sodium chloride, NaCl). How did the amount of salt water taken up by the superabsorbent compare to the amounts of distilled and tap water taken up? How would you use the previous information about salt’s effect on absorbency to explain how these comparisons make sense? You used 0.9% NaCl, because you were exploring diapers and this solution is a good approximation for the salt content in healthy human urine. There are also other dissolved solids in urine. Even the most super absorbent polymer can usually only hold up to 30 times its own weight of urine (compared to 800 times its weight of distilled water). This is actually fortunate. Consider what the diaper would look like on a baby, if it could absorb as much urine as distilled water. Was it surprising to find that sprinkling only a small amount of salt on the superabsorbent polymer-distilled water gel caused the gel to breakdown and become mushy? The discussion above provides a clue about why this happens. Salt in water lowers absorbency. Adding salt to the surface of the polymer-water gel creates a film of salty water on the surface. In order for the inside and outside of the polymer mesh to come into “saltiness balance”, water leaves the mesh. This makes the mesh collapse somewhat and destroys the integrity of the gel, which turns mushy. |
|
| Some other ideas to try out to further explore the absorbency of this superabsorber | |
Try testing other types of water like bottled water or lake water. Try to predict which ones a diaper will absorb better, based on salt content. Try other beverages such as seltzer water and sports drinks. Sports drinks are designed to replace electrolytes (like salt) that you lose when you sweat. What would you predict about a sports drink’s absorbency? Try other brands and sizes of diapers. Test to see if materials other than salt, such as baking soda, vinegar, or sugar, can release the water and un-gel a gelled polymer. |
|
| PROCEDURE 2 | |
| WHAT YOU WILL NEED | |
|
|
| In this experiment, you will test the absorbency power of a superabsorber, specifically the one in diapers that can hold many times its own weight in water. | |
| PREPARATION | |
Make a solution of 0.9% salt water by adding ½ teaspoon of salt to 1 ¼ cups of distilled water and mixing with a spoon. Use food coloring to dye some distilled water, tap water, and the salt water you just made. You can use the same color for all 3 or a different color for each. |
|
| HOW ABSORBENT IS A DIAPER? | |
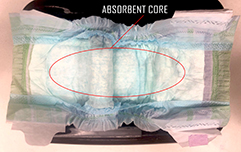
Perform this experiment outside, over a sink, or anywhere you won’t mind getting wet. You will need 3 diapers, one for each type of water you are testing. |
|
 |
|
Unfold one of the diapers and slowly pour about ¼ cup of distilled water over the center, moving back and forth so that you cover the absorbent core completely. Pick up the diaper at both ends and gently rock it back and forth for 5-10 seconds. What do you observe? Did the diaper absorb all of the water? What does the core feel like? Try adding more distilled water in ¼ - ½ cup increments, gently rocking the diaper back and forth after each addition. Has anything changed? How much of each water can you add until the diaper appears to no longer absorb anything? What does the diaper look like now? How does it feel? You can use a ruler to measure the height of the absorbed water. Try repeating this experiment with tap water and salt water in the other two diapers. Record how much of each water a diaper was able to absorb in a data table like this: |
|
|
You can design your own data table, or click on the example above |
|
Once you are done adding water to all 3 diapers, think about what you observed. Is one diaper heavier than the other? Does the diaper absorb more of a certain kind of water than others? If so, why do you think that is? What is different about the water? Now, you can use scissors to cut open the diaper you filled with distilled water and see the superabsorber. Start cutting from one end and only cut the top layer so that the bottom of the diaper remains uncut. What is inside the diaper? Is it what you were expecting? The material is safe to touch – just make sure to wash your hands afterwards! Next, use a tablespoon to scoop a bit of the material into a plastic cup. Sprinkle a bit of table salt onto the material and mix with a spoon. What do you observe happening? |
|
| OPTIONAL | |
What did the superabsorber look like before water was added? Place a diaper in a Ziploc bag. While the diaper is in the bag, use scissors to cut the diapers into approximately 2-3 inch squares. When you are done, seal the bag, shake vigorously and then look at the corners of the bag as you tilt it to one side. Keep shaking and moving around the material until you see a pile of white granules fall into the corner of the bags. Placing a bag on a hard surface and gently pounding it with your fist may also help loosen some of the material. These white granules are the superabsorber! Is it surprising that those small granules were able to cause the swelling observed in the diaper soaked with distilled water? |
|
| DISPOSAL | |
| Throw away used diapers and diaper material in the trash. Any diaper material already liquefied with salt can be poured down the drain with running water. | |
The superabsorbent material in the diaper that you saw as white granules is a polymer called sodium polyacrylate. A polymer is a special type of chemical made of many repeating parts (poly = many, mers = parts). The parts are small molecules that link together through chemical bonds, forming very long chains with unique properties. Polyacrylate’s structure is specially designed to take in and retain lots of water molecules – this is why pure sodium polyacrylate is often sold as a product called WATER LOCK. When the diaper’s superabsorber, usually a combination of sodium polyacrylate with other absorbers, soaks up water, you notice that a solidified gel forms and can swell to multiple times the original size of the powder. These observations are consequences of the polymer “locking in” water through strong interactions between the water molecules and the polymer. People usually find that a diaper’s sodium polyacrylate can easily absorb lots of distilled water, about half as much tap water, and only a small amount of salt water. The reason for this difference is the salt content. Salt interferes with the polymer’s ability to absorb water by blocking the spots on the polymer where water would be taken up. Distilled water is pure water so its maximum amount can be absorbed. However, tap water contains a number of dissolved salts, lowering the polymer’s absorbing power. The EPA recommends that the total dissolved solids in drinking water not exceed 500 ppm (0.05%). Is it surprising that such a small amount of salt can reduce the polymer’s absorbency by as much as you saw? You can see the kinds of solids dissolved in your tap water by accessing your annual drinking water quality report, an annual report compiled by community water systems as required by the EPA. To find the report online, search for “Annual Water Quality Report” and your city/state. The salt water you used was 0.9% table salt (NaCl). How effectively was this salt water taken up compared to the distilled and tap water? Does this make sense given what you now know about salt’s effect on absorbency? Since we are exploring diapers, we used 0.9% NaCl, which is a good approximation for the salt content in healthy human urine. There are additional dissolved solids in urine as well, which is why even the most super absorbent diapers can usually only hold up to 30 times the weight of the absorbing polymer (compare that to 800 times the weight of the absorbing polymer of distilled water!) What do you think happened when you broke apart the gel with salt? Just as salt water inhibits absorbency, adding a lot of salt to the gelled polymer and water forces the polymer to “unlock” the water and turns the gel back into a liquid. |
|
| Some other ideas to try out to further explore the absorbency of this superabsorber | |
What if you tested other types of water like bottled water or lake water? Can you guess which ones a diaper will absorb better based on salt content? What about beverages such as seltzer water and sports drinks? Sports drinks are known to contain a lot of electrolytes (salts). How would this effect a diaper’s absorbency? You can try other brands and sizes of diapers. You can see if materials other than salt can release the water, such as baking soda, vinegar, and sugar. |
|