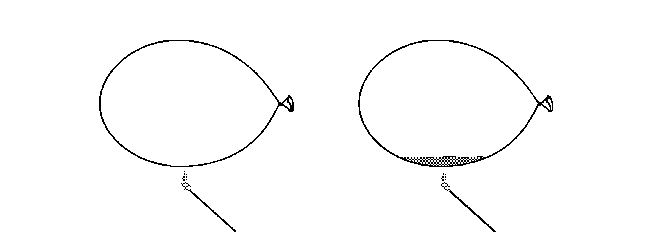
Balloons are rather fragile things. You know that they must be kept away from sharp objects. The also need to be kept away from flames. A fire can weaken the rubber and cause it to burst. However, in this experiment you will find out how you can hold a balloon directly in a flame without breaking the balloon. For this experiment you will need:
Inflate one of the balloons and tie it closed. Place 60 milliliters (¼ cup) of water in the other balloon, and then inflate it and tie it shut. Light a match and hold it under the first balloon. Allow the flame to touch the balloon. What happens? The balloon breaks, perhaps even before the flame touches it. Light another match. Hold it directly under the water in the second balloon. Allow the flame to touch the balloon. What happens with this balloon? The balloon doesn't break. You may even see a black patch of soot form on the outside of the balloon above the flame. |
|
|
|
Why does the balloon with no water break in the flame? The flame heats whatever is placed in it. It heats the rubber of both balloons. The rubber of the balloon without water becomes so hot, that it becomes too weak to resist the pressure of the air inside the balloon. How does the balloon with water in it resist breaking in the flame? When water inside the balloon is placed in the flame, the water absorbs most of the heat from the flame. Then, the rubber of the balloon does not become very hot. Because the rubber does not become hot, it does not weaken, and the balloon does not break. Water is a particularly good absorber of heat. It takes a lot of heat to change the temperature of water. It takes ten times as much heat to raise the temperature of 1 gram of water by 1C than it does to raise the temperature of 1 gram of iron by the same amount. This is why it takes so long to bring a teakettle of water to the boil. On the other hand, when water cools, it releases a great deal of heat. This is why areas near oceans or other large bodies of water do not get as cold in winter as areas at the same latitude further inland. CAUTION: Be careful when handling matches to avoid burning yourself or causing accidental fires. |
|
|