


Pure water does not conduct electricity very well. However, when certain substances are dissolved in water, the solution does conduct electricity. You can make a simple device that shows how well a solution conducts electricity. This device uses a flashlight bulb to indicate how well the solution conducts electricity. The better the solution conducts electricity, the brighter the bulb will glow.
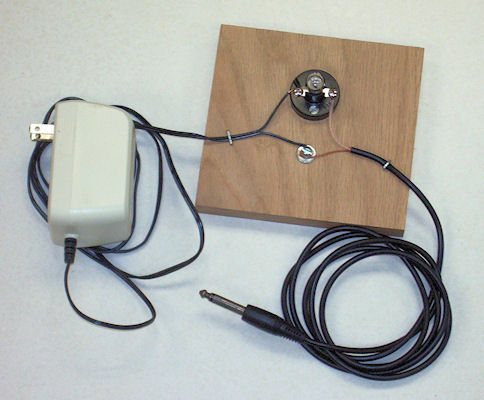
Conductivity Tester
To construct the conductivity tester you will need:
- ● a 12-volt AC adapter
- This converts the 110-volt electricity from a wall socket to safer 12-volts. It must be 12 volts AC, not DC, because DC will not work for this. You may have a suitable adapter around the house from an old device you're no longer using, or you may get one from an electronics store (e.g. Radio Shack, catalog number 273-1631).
- ● an audio cable with a 1/4-inch or 1/8-inch monaural plug on one end
- The plug will become the probe for testing conductivity. You may have an unused cable around the house. What is on the other end does not matter because it will be removed. You may also get a suitable plug-and-cable assembly from an electronics supply store (e.g., Radio Shack, catalog number 42-2381).
- ● a 12-volt flashlight bulb and socket
- The bulb will provide a visible indication of how well a material conducts electricity. You can get these from an electronics store (e.g., Radio Shack, catalog numbers 272-1143 for the bulb and 272-357 for the socket).
- ● a block of wood about 4 by 4 by 1 inch
- The electrical connections will be made on this block, and the lamp will be mounted on it, too.
- ● two 1-inch wood screws
- These hold the lamp socket to the block of wood.
- ● one 3/4-inch round-headed screw and washer
- These will be used to make an electrical connection.
- ● wire cutter and wire stripper
- These are used to prepare the electrical connections.
- ● a screw driver
Cut the plug from the end of the cord of the AC adapter. Separate about four inches of the cord into its two conductors. Remove about 1 inch of insulation from each of the conductors.
Cut the cord of the audio cable about 2 feet from the plug. Remove about four inches of insulation from the cut end of the cable. This will expose bare stranded wire wrapped around insulation that covers a center wire. Unwrap the stranded wires from the insulation and twist the strands together to make a single bundle. Strip about 1 inch of the inner insulation from the center wire.
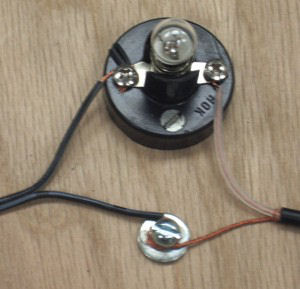
Electrical connections
Use wood screws to attach the lamp base (socket) to the block of wood. Put the washer on the round-head screw and screw it into the block next to the lamp base, but do not tighten the screw yet.
Wrap one wire from the AC adapter (it doesn't matter which) around the screw above the washer. Wrap the end of the bundled wire from the audio plug around the same screw. Tighten the screw to fasten the two wires together.
Attach the remaining wire from the AC adapter to one of the terminals of the lamp base. Attach the remaining wire from the audio plug to the other terminal of the lamp base.
Screw the 12-volt flashlight lamp into the lamp base.
To make the connections more secure, you can use a heavy staple to hold each of the two wires to the wooden block.
The conductivity tester is now complete and ready to use. To test that it works properly, plug the AC adapter into an AC outlet. The lamp will not light. Touch the audio plug sideways to a piece of metal, such as a coin. When the two metal conductors of the plug are shorted by the coin, the lamp will glow brightly. The bright glow indicates that current is easily flowing through the piece of metal.
Testing a solution
Put some water into a cup. Insert the end of the audio plug into the water. If you use distilled water, the lamp will not glow. If you use tap water, the lamp may glow dimly, if at all. If it glows, it shows that the tap water conducts electricity only poorly. Add some table salt to the water and stir the mixture. The lamp will glow brightly when the plug is put into the solution, because salt solution conducts electricity very well, almost as well as metal.
You can investigate different materials from around your house to see how well they conduct electricity when mixed with water. Some things to try, in addition to salt, are sugar, baking soda, shampoo, laundry detergent, rubbing alcohol, and antacid tablets. Anything that dissolves in water can be tested. In order to avoid mixing the materials you're testing, be sure to rinse the plug in water and dry it before testing a different substance. Do not put the plug in a solution for more than 10 to 15 seconds, because doing so will cause the plug to corrode rapidly. Keep a record of which substances conduct electricity well, which conduct poorly, and which do not conduct at all.
Sometimes, mixtures of substances conduct differently than the separate substances. As an example, test the conductivity of vinegar. Then test the conductivity of laundry ammonia. Then, pour a little ammonia into the vinegar and test the mixture. You will see a big difference between the separate substances and the mixture!
An electric current is a flow of electrical charge. When a metal conducts electricity, the charge is carried by electrons moving through the metal. Electrons are subatomic particles with a negative electrical charge. When a solution conducts electricity, the charge is carried by ions moving through the solution. Ions are atoms or small groups of atoms that have an electrical charge. Some ions have a negative charge and some have a positive charge.
Pure water contains very few ions, so it does not conduct electricity very well. When table salt is dissolved in water, the solution conducts very well, because the solution contains ions. The ions come from the table salt, whose chemical name is sodium chloride. Sodium chloride contains sodium ions, which have a positive charge, and chloride ions, which have a negative charge. Because sodium chloride is made up of ions, it is called an ionic substance.
Not all substances are made up of ions. Some are mode of uncharged particles called molecules. Sugar is such a substance. When sugar is dissolved in water, the solution does not conduct electricity, because there are no ions in the solution.
Some substances that are made of molecules form solutions that do conduct electricity. Ammonia is such a substance. When ammonia dissolves in water, it reacts with the water and forms a few ions. This is why laundry ammonia, which is a solution of ammonia in water, conducts electricity, but not very well.
Sometimes, when two different solutions are mixed, the substances they contain react with each other and form ions. This is what happens when ammonia and vinegar are mixed. An ammonia solution contains only a few ions, and it conducts electricity only poorly. A vinegar solution also contains only a few ions and conducts only a little electricity. But when these solutions are mixed, the ammonia reacts with the acid in vinegar (acetic acid), and they form a lot of ions. This is why the mixture of ammonia and vinegar conducts electricity very well.